k-bob
Well-known member
- Joined
- Jul 29, 2009
- Messages
- 2,376
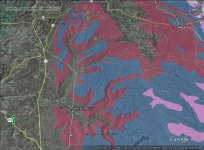
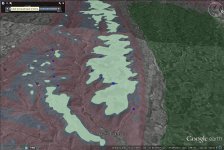
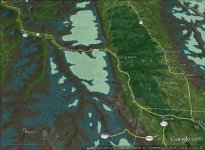
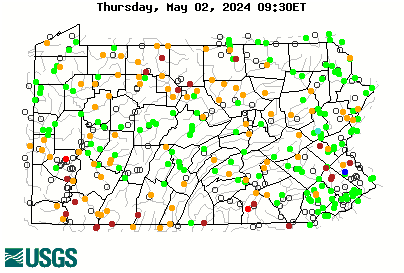
the ebfc report linked post 60 spells it out nicely, noting that swampy headwaters add acid and will increase aluminum. can see swampy headwaters in sat maps of course, and also usgs maps mark them well...
"One characteristic of acid waters is the presence of elevated concentrations of dissolved aluminum. Aluminum is the third most abundant element in the earth’s crust and under buffered soil conditions remains essentially immobile. Acid rain, however, can increase the mobility of aluminum and greatly increase the concentration transported into streams. The elevated levels of aluminum can be toxic to fish and other aquatic organisms; the collection of aluminum on their gills limits the intake of oxygen and other important nutrients." ...
"The concentration and speciation of aluminum in streams can vary, being dependent on the chemical composition of soils, geology, the pH of infiltrating water, and the presence of natural tannin-based (bog) acidity in the headwaters of a stream. The equilibrium concentration of aluminum in water is inversely proportional to pH below a pH of about 7 SU, such that as pH decreases aluminum concentrations increase. Aluminum concentrations also increase directly above a pH of about 9 SU, but this is seldom a problem in natural waters. The solubility increases dramatically below a pH of 4.5 SU, which is incidentally the approximate pH of acid rain in eastern Pennsylvania and East Branch Fishing Creek."
http://www.wehydro.com/images/2007fc.pdf
(emphasis added)
"One characteristic of acid waters is the presence of elevated concentrations of dissolved aluminum. Aluminum is the third most abundant element in the earth’s crust and under buffered soil conditions remains essentially immobile. Acid rain, however, can increase the mobility of aluminum and greatly increase the concentration transported into streams. The elevated levels of aluminum can be toxic to fish and other aquatic organisms; the collection of aluminum on their gills limits the intake of oxygen and other important nutrients." ...
"The concentration and speciation of aluminum in streams can vary, being dependent on the chemical composition of soils, geology, the pH of infiltrating water, and the presence of natural tannin-based (bog) acidity in the headwaters of a stream. The equilibrium concentration of aluminum in water is inversely proportional to pH below a pH of about 7 SU, such that as pH decreases aluminum concentrations increase. Aluminum concentrations also increase directly above a pH of about 9 SU, but this is seldom a problem in natural waters. The solubility increases dramatically below a pH of 4.5 SU, which is incidentally the approximate pH of acid rain in eastern Pennsylvania and East Branch Fishing Creek."
http://www.wehydro.com/images/2007fc.pdf
(emphasis added)