pcray1231
Well-known member
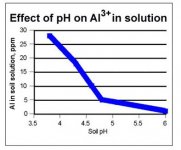
Fair enough. From a farming site, the image below is of Al in a soil "solution". i.e. a measure of the Al that's been separated from it's oxide or silicate base and thus sitting in the soil for moisture to pick up. You need acids to separate it and make it "available" for water to pick it up. You do not have free Al in basic soils.
More basic water coming in contact with this free Al in the soil will dissolve more of it, though.
So it may be that the "limiting factor" in streams is the availability of free Al rather than the ability of the water to dissolve it.
More basic water coming in contact with this free Al in the soil will dissolve more of it, though.
So it may be that the "limiting factor" in streams is the availability of free Al rather than the ability of the water to dissolve it.