Aluminum is an interesting one, IMO.
It's the 3rd most abundant element in the Earth's crust (behind oxygen and silicon). There is no shortage of it in the soil.
It is not found as a soluble metal very often, though, because it easily forms oxides and silicates in nature. Acid breaks apart said oxides and leads to soluble aluminum. Take a piece of aluminum foil and put it in some HCL and watch it bubble. The surface is Al-oxide which is being attacked, and once removed, the next layer down forms Al-oxide and likewise gets attacked, till its all eventually powder sitting at the bottom of the beaker. The acid doesn't attack the Al itself, it attacks the Al-oxide. That Al powder, though, is not very soluble in acidic fluid, it's soluble in a basic fluid!
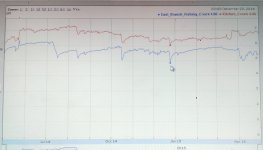
But rainwater contains virtually no aluminum, and acidic water doesn't carry much. Hence, soluble aluminum DECLINES during runoff events themselves, which is the most acidic period in a stream.
It comes from groundwater. Out of springs. Generally from water in the ground that was once more acidic, thus precipitated the aluminum, and became more basic, thus picked it up in solution. It's a decent measure of acid sources in the soil itself.
Of course, acidic soil can be the result of acid deposition from acid rain, which acidifies the soil, leaches aluminum from the soil, then becomes more basic as it hits bedrock and flows to a spring. Heavily conifered watersheds can do the same, as pines make acidic soil. It can also be natural geologic formations like pyrite, where water passes through and becomes acidic, leaches out aluminum from the soils just downstream, and then slowly becomes more basic. Or, it can be deep mines which essentially magnifies the same process above.
Your highest aluminum periods will be when groundwater flows are strong and runoff contribution is minor. Think April when it hasn't rained for a week or so. As such, it'll be higher in forested watersheds, where there is more groundwater and less runoff. And it'll be higher in streams which are more acidic in their base state, whether that base state is natural or man-degraded.