k-bob
Well-known member
- Joined
- Jul 29, 2009
- Messages
- 2,371
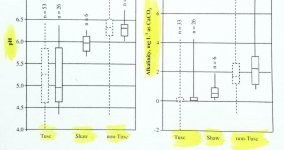
striking to see how much pa trout streams seem to be affected by acid rain as buffered by various local geologies. potter county, with mostly high buffering bedrock, has about 100 class a streams (not counting streams listed across 2 counties, for example "mckean-potter"). forest county, with mostly low buffering bedrock has about 5. adjust for the larger size of potter, and 1000 sq miles of potter on average has ten times more class a streams vs 1000 sq mi of forest.
I am sure there are other things going on here, how much surveying done, etc., but if potter had forest's bedrock makeup things would be different there. Pa rain has become less acidic, but apparently stream water acid reductions have a lag time after this.
I am sure there are other things going on here, how much surveying done, etc., but if potter had forest's bedrock makeup things would be different there. Pa rain has become less acidic, but apparently stream water acid reductions have a lag time after this.